Boat Nerd: Lithium Batteries – Why? Part 2 Science

Disclaimer: This article is offered as information only. We strongly recommend that boat owners do NOT install lithium-ion batteries themselves, but only have authorized dealers do it. Once installed, any changes or modifications must be done by authorized dealers. These batteries are managed by specific BMS devices calibrated for each specific installation, and tampering with, or changing out parts may lead to a fire – which generally can’t be controlled.
Last issue, The Boat Nerd, Mike Wheatstone, introduced us to lithium batteries and explained why they are important. This time, we dig deeper into the technology inside them.
The Science Behind Lithium Batteries
All lithium-ion batteries, as their name suggests, are based on the movement of lithium ions driving the reactions within the battery.
Think of a battery as consisting of:
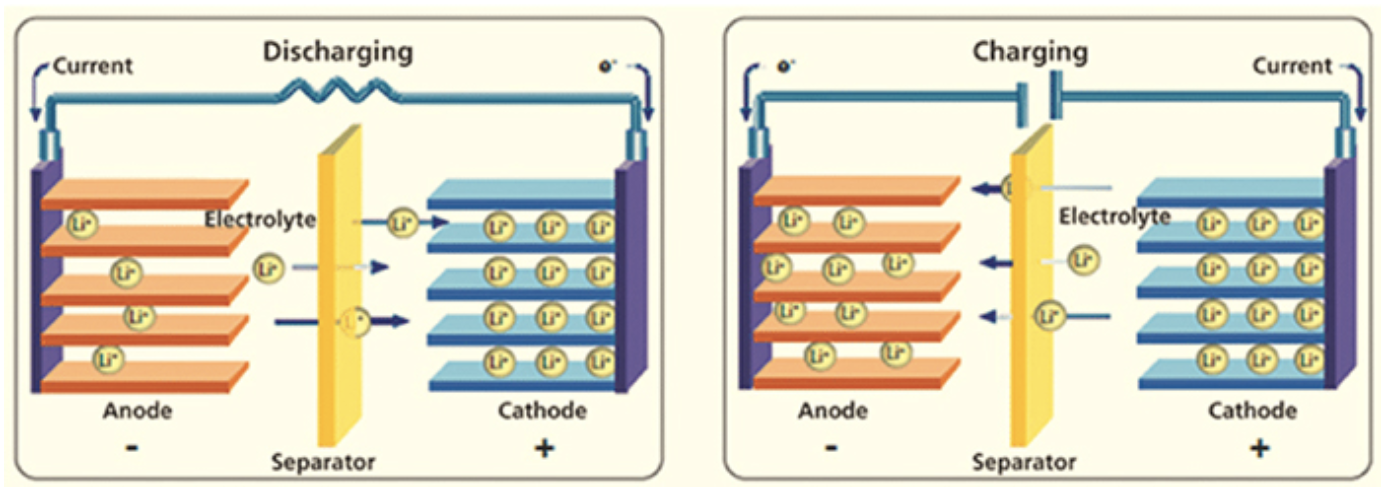
1. Two electrodes that can absorb lithium ions at either end of the cell. One of the electrodes is called the Anode and is made of carbon, typically graphite. The other electrode is the Cathode and made of a metal oxide.
2. A liquid electrolyte sits between the two electrodes and carries the positive charge lithium ions between the anode and cathode if being charged and visa-versa under discharge. More recently a solid polymer electrolyte is replacing the liquid one resulting in a lighter and safer battery.
3. A separator in the middle of the cell that blocks the flow of electrons within the battery but allows the passage of ions.
The type of lithium-ion battery is dependent of the chemical composition of the cathode, for example lithium cobalt oxide, lithium manganese oxide, or the one will are most interested in, Lithium Iron Phosphate (LiFePO4). The battery terminal voltage also varies slightly with the chemistry involved.
Lithium cobalt oxide batteries have the highest energy density and are what you will find in mobile devices where long charge life, small size and light weight are paramount concerns. However they are also the most thermally unstable. You may remember stories of Boeing’s 787 Dreamliner battery fires or some Samsung mobile phones catching fire.
The phosphate in LiFePO4 can tolerate high temperatures and makes for a very thermally stable battery. While Cobalt based chemistries have a higher energy density, LiFePO4 with its lower but still very respectable energy density is the only chemistry that should be used on boats due to its thermal stability. Fires in cobalt lithium batteries are extremely hard to extinguish. Not what you want on a boat.
The terminal voltage of LiPo cells is 3.7V compared to that of LifePO4, which is 3.2V per cell.
What Reaction Takes Place in a Lithium Cell when Charging or Discharging?
(You can skip this section if you’re not interested in the gory details)
Electrodes in a lithium battery work because the lithium ions can be held in the lattice structure of the electrode material without materially disturbing it. To preserve electrical neutrality, each positive lithium ion is coupled with a negative electron within the structure of the electrode.
Upon fabrication a lithium-ion battery is in a completely discharged state. All the lithium ions (and attendant electrons) have been absorbed within the cathode. Before any electricity can be obtained from the battery it must be charged.
During charging, the charging source causes an oxidization reaction to occur at the cathode whereby it loses some of its negatively charged electrons. To keep electrical neutrality in the cathode, an equal number of the positive charge lithium ions stored in the electrode, migrate into the electrolyte solution. These ions travel through the electrolyte to the anode where they are stored in the graphite lattice. The electrons pulled off the cathode by the charging source are combined with the migrated lithium ions in the anode, preserving electrical neutrality.
During discharge the opposite happens. When an external load is connected to the battery, electrons flow from the anode releasing the ions that were tied to them, into the electrolyte to travel to the cathode. At the cathode the electron are then tied with the ions again to preserve electrical neutrality. Without the external electrical connection to the electrodes, no electrons are free to travel and there is no reaction in the battery. It’s the negative electron movement through the external circuit that allows the balancing positive ion movement through the electrolyte.
When the cathode has absorbed all the lithium ions it can, the battery is flat and no further energy can be taken out of it. The battery must then be recharged by connecting an external voltage source, pushing the lithium ions from the cathode back to the anode.
The electrolyte is typically a mixture of lithium salts in a mixture of solvents. The dissolved lithium salts create free lithium ions in the electrolyte. These electrolyte ions mean the ions released from one of the electrodes do not have to travel the entire path to the opposite electrode to complete the circuit. During discharge, ions leaving the anode enter the electrolyte while ions in the electrolyte near the cathode surface are absorbed into it. During charging the reverse happens.
Lithium-ion batteries get their high energy density because Lithium is molecularly small and light resulting in lots of it being able to be stored in the electrode material lattices. For example one lithium ion can be stored with six carbon atoms in the graphite. The greater the number of ions stored in the material, the more ions are available to travel between the electrodes and a corresponding greater electron flow in the external circuit.
The movement of ions between the electrodes in a single lithium-ion cell occurs at a voltage of 3.2V or higher, depending on the chemical makeup of the cathode. Contrast this to the 1.5V typically obtained from a single alkaline cell or the 2V from a single Lead-acid cell. Thus for marine use we typically stack four lithium cells in series to get a nominal 12V battery.
Next time, Part 3 looks at the packaging of lithium batteries, battery management systems, implications for insurance and charging characteristics.