Electric Propulsion, Part 6: How Batteries Work

This seems as good a time as any to look into how batteries work. They all basically work the same, though the internal chemistry for storage may be different. A note to all experts out there, this is extremely simplified.
A battery has one kind of material with atoms that want to shed their electrons, another type of material with atoms that want to gather electrons, and in between them a material that facilitates a chemical reaction that sets the electrons free so they can go and collect on the other material.
The Simplest Battery
As a kid you may have made a battery made out of a lemon, a nail and a copper penny. The zinc in the galvanized nail wants to get rid of electrons, the copper in the penny wants to gather them, and the lemon juice is the material in between.
The nail and the penny are called the electrodes of the battery. The nail is the negative electrode — the anode — the penny is the positive electrode — the cathode — and the lemon juice is called the electrolyte.
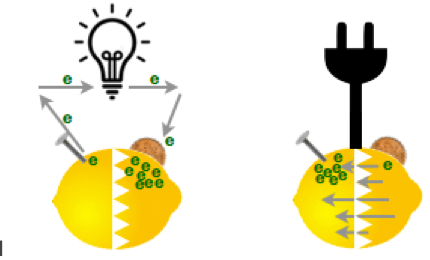
In a rechargeable battery, like the kind you want in your boat, the chemicals are a lot more complicated and there is also a one-way barrier. Hooking up a motor between electrodes creates a chemical reaction with the electrolyte that makes the electrons flow through the motor to get from the anode to the cathode (this is called electricity) and the motor spins. When you plug it into a recharger, the one-way barrier lets the electrons go directly back through the barrier to the other electrode.
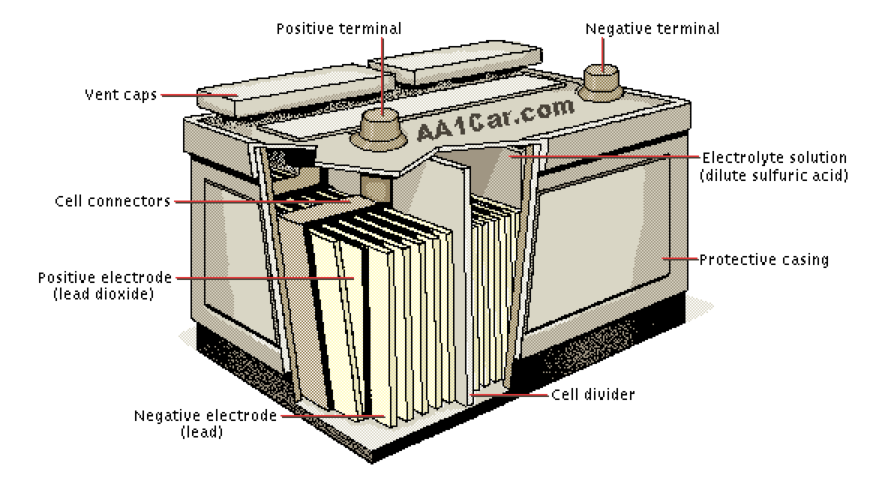
As you can imagine, the chemical reaction of a lemon, nail and copper coin doesn’t generate a lot of electricity. Surprisingly, neither do the chemical reactions of the latest and most efficient battery chemistries — it’s only about 2 volts to 3.6. volts.
We’ll learn more about how they produce lots more voltage than that in the next sections.
This report by Jeff Butler is courtesy of Plugboats.com.