Tech Talks - Corrosion

If there is one thing more confusing and difficult to understand than electricity, it’s chemistry. So, when a boater hears that corrosion is an electrochemical process, it’s no wonder both sides of his brain begin hurting. Protons, electrons, neutrons, covalent bonding, ionic bonding, valence shells – enough! Just slap a new zinc on there and hit the water, right?
Actually, in a way, that’s not bad advice for a Nordhavn. But, before getting into how we protect Nordhavns from corrosion, let’s back up and take a more general look at the problem of corrosion on boats.
Corrosive Influences
There are three types of corrosion for boaters to be aware of: electrochemical, electrolytic and galvanic. And four things must always be present for corrosion to occur: an anode, a cathode, a metallic path for electrons to flow and an electrolyte.
Most boaters have at least heard of galvanic corrosion, but the other two might not be on their radar unless they’ve actually had issues that involved electrochemical or electrolytic corrosion.
A good example of electrochemical corrosion (also known as single-metal of self corrosion) is an isolated brass fitting immersed in seawater. Brass is an alloy of copper and zinc, so zinc becomes the anode, copper the cathode, seawater is the electrolyte and the metals in the alloy are obviously touching to provide a path for electron flow.
The electrons flow from the zinc to the copper and an ionization reaction takes place at the anode (zinc) causing it to dissolve – this dissolving is what we call corrosion. Thankfully electrochemical corrosion is a very slow process and is typically not a major concern over the lifetime of a boat.
Electrolytic corrosion (commonly called stray current corrosion), on the other hand, can do real damage in a matter of just days or even a few hours. Some like to call this electrolysis, but that term is actually something else – better to just call it stray current corrosion. This type corrosion relies on an outside “stray” source of electrical current, such as a loose positive DC cable dangling in the bilge water or possibly an electrical fault on a nearby boat at the dock.
Problems can range from accelerated zinc anode wastage to massive corrosion of props, shafts, thru-hulls and other underwater metals. The tell here is that it happens fast – much faster than electrochemical or galvanic corrosion.
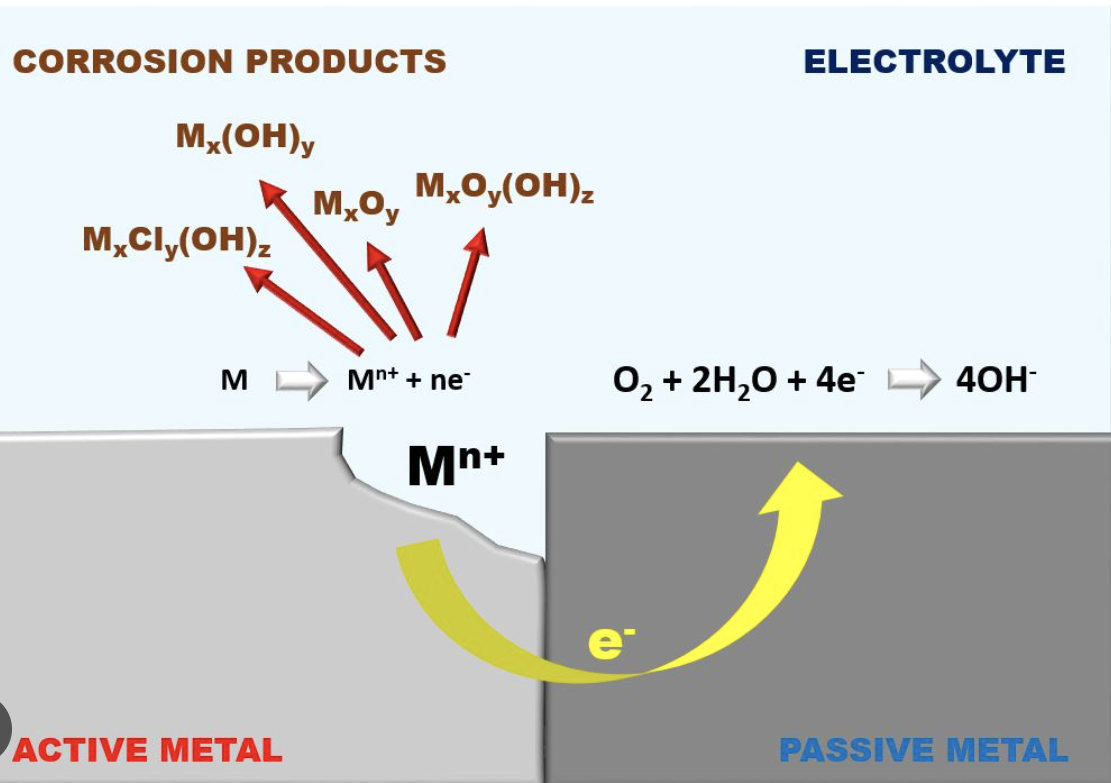
Now we reach galvanic corrosion – the biggest concern for boaters. Galvanic corrosion is just a natural consequence of boating. All the components are there: dissimilar underwater metals to create anodes and cathodes, seawater as an electrolyte and common metal connections within the boat’s machinery and wiring.
This sets up a basic galvanic cell where the more anodic (active) metal will waste away while the more cathodic (noble) metal will remain intact. A quick look at the galvanic series will show how the metals on the top (anodic) will suffer while the metals on the bottom (cathodic) will be protected. The further they are apart, the more potential there is for damage.
Think stainless steel propeller shaft (like Type 316 passive) meets bronze propeller – bye-bye bronze propeller.
Protection From Self
In the case of a Nordhavn (and pretty much any other boat), we’re left with a situation where we have to protect the boat from itself. We like stainless steel propeller shafts because they are strong, durable and can take the beating of transferring power from the propeller to the hull.

We like bronze propellers because they are also strong and durable – and they are cost effective (a large stainless steel propeller would be very expensive). But put the two together under water and we’ve got problems.
In order to protect the boat from itself we actually go back to the galvanic series and find an inexpensive solution right there near the top: zinc. Most boaters have heard of cathodic protection and sacrificial anodes, which is exactly what we’re talking about.
By adding zinc anodes to the mix of underwater metals we’re creating a front line of little gray soldiers who will sacrifice themselves in order to protect our relatively cathodic stainless steel shafts, bronze propellers, thru-hull fittings and other underwater metals. As long as we remember to replace our zinc anodes after they are 50% depleted we shouldn’t have anything to worry about.
One point to bring up here is the strategy of bonding, which is necessary for this kind of boat-wide cathodic protection to work. Cathodic bonding describes electrically connecting all of the underwater metals together, typically using a system of copper bars that run along the inside of the hull and bonding wires to attach individual components like thru-hull fittings, prop shafts, rudder struts and zinc anodes to the copper bars. This electrical connection is absolutely necessary for the zincs to protect the rest of the underwater metals.
However, it is possible to provide local cathodic protection by bolting underwater zincs right onto the metals they are intended to protect (collar zincs for shafts, propeller hub zincs for propellers, special zincs for keel coolers, etc.) and not using a bonding system with centralized zincs to protect the entire system.
And some metals, like quality bronze thru-hulls, can provide decades of service with no protection at all. As long as they are isolated they are only subject to simple electrochemical corrosion, but the rate of corrosion is so slow that it is not an issue.
Nordhavns use a bonding system with an appropriate number of external hull zincs as well as some individual collar and hub zincs for the shafts and props.
Protection From Others
Plugging a boat into shore power can be like using a public toilet without using a seat cover. Without protection you might catch something. The problem centers around the green grounding wire. The grounding wire is necessary for circuit breakers to properly work to clear electrical faults and prevent people from being harmed, but just by being there it makes it possible for problems on other boats to cause issues on your boat.
On the not-so-bad side this can result in accelerated wastage of your zincs as they struggle to protect the metal on someone else’s boat as well as on yours. On the oh-so-bad side it can result in serious corrosion of your underwater metals or dangerous voltages being brought onto your boat.
For smaller boats the most common solution is typically a galvanic isolator placed in series with the green grounding wire. This device will block small galvanic currents from coming aboard and effectively isolate your underwater metals from the rest of the boats on the dock. In the event of a serious electrical fault, it will still allow the grounding wire to do its job in order to provide protection for personnel.
Large boats will many times have what is called an isolation transformer, which is a large electrical device with windings that uses induction to pass current rather than a physical connection with wires. This basically creates an independent power source for the boat with its own grounding conductor, so there is no physical grounding wire connection between the boat and the dock.
This is the ultimate solution because it makes it impossible for any kind of issue, galvanic or otherwise, to be brought aboard on the green grounding wire – because this connection to the dock no longer exists.
All of the larger Nordhavns include an isolation transformer for the main ship service shore power connection and a galvanic isolator for the dedicated air conditioning shore power connection (if included). The smaller boats, like the N40 and N43, are typically ordered with only galvanic isolators.
One trend that seems to be on the rise, especially with larger boats, is the inclusion of shore power converters that can accept power within a wide range of voltages and frequencies – these are ideal for boaters who frequent foreign ports (like many Nordhavn owners do). In terms of electrical isolation from the dock these devices do the same thing as an isolation transformer.
This article only scratches the surface on the topic of boat corrosion. The American Boat & Yacht Council (ABYC) actually offers a certification course on this subject, which is a great opportunity for those who want to learn more. The text for the course is The Boat Owner’s Guide to Corrosion by Everett Collier, which is excellent and highly recommended for those who want to learn more on their own.
Another good source of information on this topic is www.qualitymarineservices.net, which includes a number of photos and additional information on boat corrosion.